The Human Motion Institute
Sylvia Lawry Centre for MS Research e.V.
Scientific Director: Martin Daumer PhD
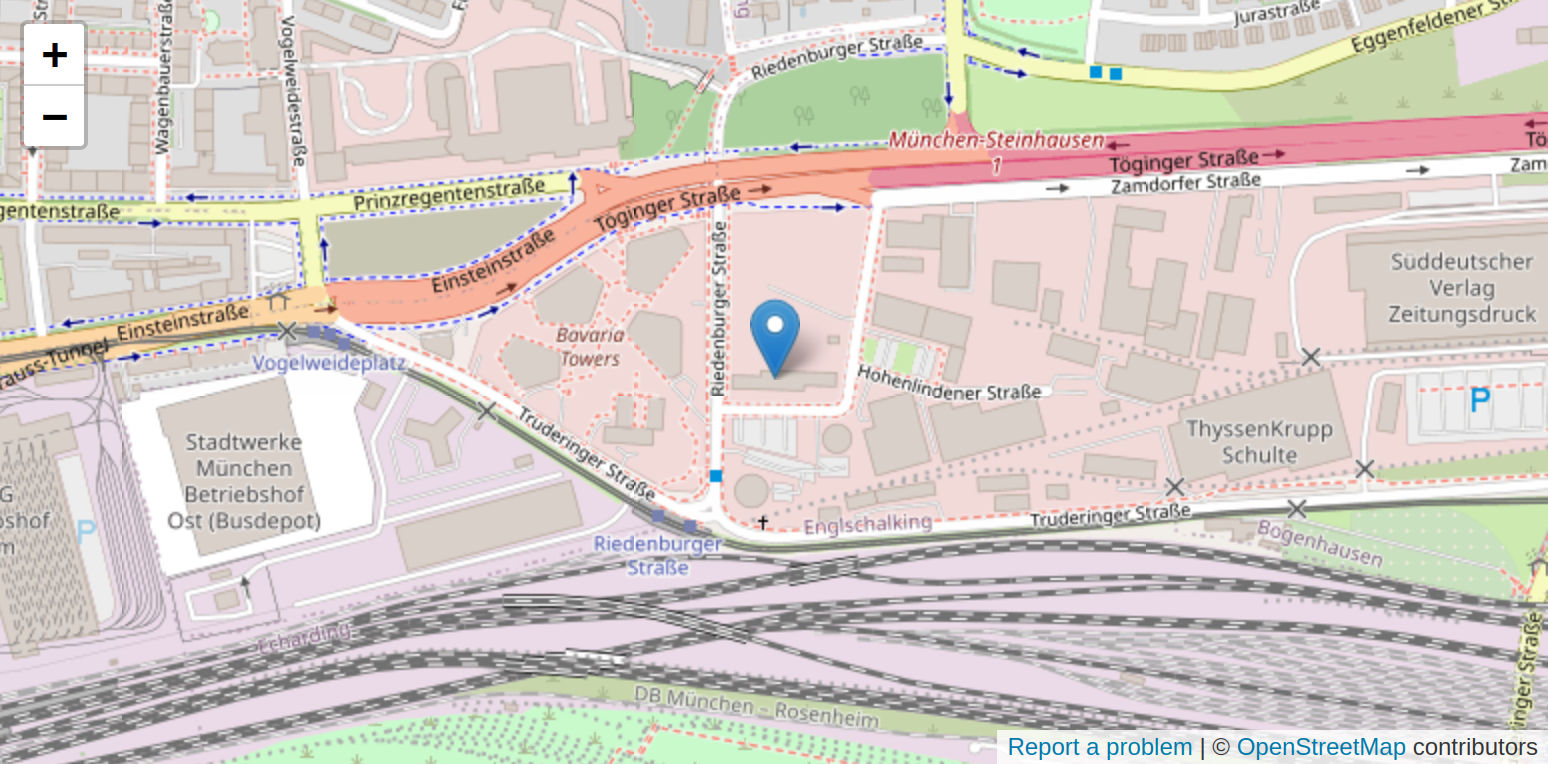
Hohenlindener Str. 1
81677 Munich
Germany
Phone: +49 89 2060 269 50
Fax: +49 89 2060 269 51
E-mail:
info@humanmotioninstitute.de
Website:
humanmotioninstitute.de